 (Image credit: Illustration by Kevin Craft)
(Image credit: Illustration by Kevin Craft)
- Written by Futurity News
How H2 Becomes the Molecule that Make the Universe
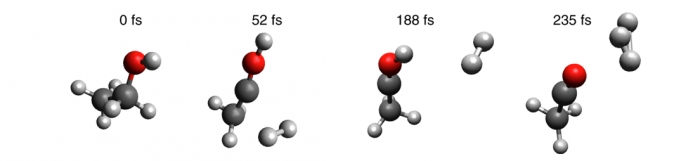
High-speed lasers are helping to shine a spotlight on the unusual chemistry of the molecule that made the universe, Trihydrogen, or H3+.
H3+ is prevalent in the universe, the Milky Way, gas giants, and the Earth’s ionosphere. The lab of Marcos Dantus, a professor in chemistry and physics at Michigan State University, is also creating and studying it. The researchers are using ultrafast lasers—and technology Dantus invented—to begin to understand the chemistry of this iconic molecule.
“Observing how roaming H2 molecules evolve to H3+ is nothing short of astounding,” Dantus says. “We first documented this process using methanol; now we’ve been able to expand and duplicate this process in a number of molecules and identified a number of new pathways.”
The research results are published in this article.
Read the full article at Futurity News.
Visible Legacy Comment
Tech Scouts should keep an eye on the development of femtosecond lasers. The results presented in this Communication confirm the prevalence of roaming H2 molecule mechanisms in the formation of H3+. This study reveals chemistry that is relevant in terms of the universe’s formation of water and organic molecules. The PI believes that secrets it could unlock, from astrochemical to medical, are endless. Dr Dantus, a Packard Foundation Fellow, is also the founder of Biophotonic Solutions Inc. (BSI), a Michigan State University spin-out venture which develops technology to automate ultrafast laser pulse techniques.
Additional Info
-
Navigator:
 Explore the map in Navigator
Explore the map in Navigator - Widget:
- Caption: Map under development
Related items
- The future of health care is in our cells
- Federal funding will help WSU professor develop technology to recover rare earth elements
- Unlocking the brain: Peptide-guided nanoparticles deliver mRNA to neurons
- Scientists Get to the Bottom of COVID’s Worst Pediatric Complication
- WSU-inspired national gene-editing task force begins work
